28 February 2007
Global Warming??
26 February 2007
Thermohaline Circulation Shutdown!?!?
21 February 2007
The Global Warming Debate.
Personally, my standpoint on the whole global warming debate is that, the general public already knows that global warming, is essentially just that. I think that the focus of the media and the government should be focused on educating the general public on exactly how, as an individual, one can help the situation out. For example, in the movie Al Gore kept giving horrifying statistics and facts, but didn't really offer a way to connect a reaction that I think would be important to our main goal--to reduce greenhouse emissions. Whether or not the facts are exactly true or whether the predictions made by scientists are fully supported, there is the chemistry behind all of our daily processes that does go to show that their worries stem from something.
While I was looking online to find some of the opposing views to the Global Warming hype, I stumbled across this article written by Capitalism Magazine about how the public is forced into accepting the views that the scientists and media has due to the fact that no one will allow a public disclaimer about how the global warming hype might not be as important as we originally thought. It is very interesting to hear other sides to the story, especially from someone who is not a scientist and only a journalist, but those people are out there.
19 February 2007
Carbon Dioxide Bubbles in Ice Cores

Chapter 4 mentions constructing past carbon dioxide profiles from the air trapped in ice cores, which I thought needed more explanation. Ice forms as new snow accumulates on top of old snow increasing the pressure. The increased pressure forms the crystalline lattice, which consists of open air spaces where carbon dioxide can be trapped during the time period of formation. With the aid of dating techniques, scientists can date each ice deposition and determine the amount of carbon dioxide present at that time period. The graph above shows how the amount of carbon dioxide and aerosols in the atmosphere was correlated to temperature (higher carbon dioxide concentrations equals higher temperatures) for the Vostok Ice Core in Greenland. For more information on ice cores and their use as paleoclimate proxies visit this page.
What do you do while driving? It may be more harmful than you think. Transportation devices as a whole still contribute almost half of the nitrogen oxides (NOx) introduced into the atmosphere today. Nitrogen oxides contribute to acid rain, the greenhouse effect, deterioration of the ozone layer and smog. The catalytic converter present in most modern cars changes nitrogen oxides back into nitrogen and oxygen using unburned gasoline. One downfall to these catalytic converters is that they only work on warm/hot engines. This means that, once a car is started, until the time the engine is “warm”, the tailpipe is essentially, emitting high levels of pollutants to the environment.
In this lab, vehicle exhaust is tested for both nitrogen oxides and particulates when the car is cool and when the car is warmed up. Since NO2 absorbs visible light, standard calibration curves for NO2 and nitrogen absorbing solutions were made. Also, using a SMPS system, that measures particulates in the air, particulate output and NOx output were assessed for seven different vehicles.
The results are as follows. From our results of hot vs. cold starts, we were expecting to find higher concentrations of NO2 on “cold starts” for each car, and lower concentrations for “hot engines”. It is interesting to note that the cars do not all exhibit the same behavior over time (the cool exhaust contains less NOx, or vice versa). While on day two of collecting data from cars, 3 of the 4 cars tested did follow our predictions (the 4th car however was the extremely old corolla, most likely not having a catalytic converter), almost the entire opposite trend happened on the first day of car sampling. Therefore according to our data, no exact conclusion could be drawn regarding the catalytic converter assumption that lower emissions of NO2 would result from a warm engine. In order of highest to lowest cold start NOx emissions the cars are as follows: Jetta, Corolla, Civic, Villager, Saab, Alero, and Echo. As for the hot, the cars rank Corolla, Saab, Villager, Alero, Civic, Echo, and Jetta. The Villager, Jetta and Civic produce much lower concentrations once they have warmed up, while the Corolla almost appears to have a malfunctioning catalytic converter.
The data from the SMPS was also analyzed and graphed, showing total particle numbers and particle volumes.
All the cars except the Jetta and Civic have greatly reduced particulate emissions once they are warm.
In conclusion, it seems that the production of nitrogen oxides in vehicle emissions is very unpredictable. However, once cars are warm they tend to greatly reduce their particulate emissions.
indoor air pollution
Vehicle Emissions Experiment 2-12-07/2-14-07

When a cars engine runs it reacts N2 and O2 during combustion under high heat creating NO as a by product. NO is a harmful chemical to be released into the air because it can undergo further chemical reactions leading to NO2. Together, NO and NO2 are referred to as NOx. These chemicals are important because they are involved in numerous photochemical reactions that lead to the creation of low altitude ozone O3, which is a primary component of smog. NOx can also react to create acid rain. According to the EPA (2003), vehicles contributed to 55 % of the total man made source of NOX emissions (http://www.epa.gov/air/urbanair/nox/index.html). We all drive cars, so each of us has a level of responsibility in creation of smog and acid rain; it is important to us to understand just how much Nox is being released by our cars.
Particulate matter is also created by the engines mechanical processes and released in emissions. Particulates also contribute to smog and therefore contribute to health problems correlated with this phenomenon. Some studies have even correlated increased levels of smog to death rate (Baird and Cann, 2005). The sizes of particles are important because the small, man made particles are inhaled directly into lungs; our bodies are not adapted to processing or filtering them or otherwise protecting us. Larger particles can be trapped by our bodies, and are less harmful to the environment because they do not stay in the air as long before settling out. The size and the amount of particulate matter that is released by our vehicles was also a focus of the study.
In order to investigate these two common pollutants from car emissions we conducted an experiment to identify the concentration of NOx and the size and concentration of particulate matter in seven different car models. The cars that were tested ranged in age from 1979 to 2001 and included small models such as a Corolla and Jetta. The NOx was tested by collecting air samples directly from the tail pipe of the running cars. Air samples were mixed with an NO absorbing solution that then reacted to give us a measurable amount of color. A UV vis-spectrophotometer was used to measure this difference in color. By creating a set of standard solutions with known concentrations that were measured by the UV vis- spectrophotometer a calibration curve was calculated. The concentration of the NOx in the car emissions could then be visually compared to the standard solutions and a concentration was calculated in units of parts per million (ppm). This tells us how many particles of NOx there are in relation to all the particles in the air. The particulate matter was measured separately using a Scanning Mobility Particle Sizing (SMPS) device. This device calculates the concentration and size of particulate matter using several pieces of machinery and a computer.
Figure 1 illustrates NOx concentration data from four of the cars that were tested. We observed that total NOx generally starts out at a relatively high range, and decreases for most cars after warm-up. This trend likely results from the fact that all cars that we tested use catalytic converters designed to reduce emissions, which do not begin to work until the cars are warm. Newer cars were built under emission regulations that have become increasingly more stringent. This is supported by the data that showed overall NOx was the highest in older vehicles and the lowest in newer vehicles.
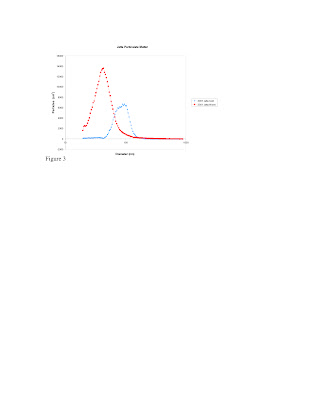
The data from Scanning Mobility Particle Sizing (SMPS) system of the 2001 Jetta and the 1979 Corolla (Figure 2) show the amount of particles at the range of sizes from 0-1000 nm in diameter. The important thing to notice is that for the Corolla the majority of particles are released before the car has warmed up and before the catalytic converter starts to work. For the Jetta the opposite is true, the majority of the particles are released after the car has warmed up. The sizes of the particles are slightly smaller also in the Jetta than the 100 nm particles released during the cold start of the Corolla. The regulation of large particulate matter in the newer Jetta might be allowing for the smaller particles to be released and go less noticed.
All of the data helps us to make observations about the composition of car emissions and connect it directly to creation of air pollution. As the world continues to modernize man made pollutants are becoming more noticeable and problematic. Specifically, increased vehicle emissions of NOX and particulates are polluting most moderately sized cities. Understanding how cars release these harmful pollutants increases our awareness of the issue and hopefully will help us to create future solutions
14 February 2007
Are We Really Conscious of the Air We Breath?
As I was reading my weekly Time Magazine, this week had an article on "Greenhoouse Airlines" and how 1.6% of greenhouse gases is due inpart to airplanes. It was interesting because it also mentioned that although this was a small percentage, the carbon released was at higher altitudes than ground level and thus had a greater warming effect. Apparently, long flights are equivelent to months of driving SUV's. That's somehting that I am sure not many people have thought about. The article also mentioned how the Prince of Wales made a public effort to cut back on his flights last year. It just makes me wonder how many more people might cut back on their flights if they were made conscious of the decisions they make.
Time Magazine, "Greenhouse Airlines" by Brian Walsh; February 12, 2007
www.time.com
13 February 2007
Pint-sized car engine
I mean if that doesn’t sound interesting then maybe some of the stats will blow your mind. Like that this new engine will go 30% farther on a gallon of fuel than a typical gas engine. And, if all cars had this engine today, we would be using 30 billion gallons of gasoline..yea GASOLINE, NOT OIL. Also, the emissions would be much cleaner due to the ethanol supplementation. That’s huge!
Read up on this at the following website: http://web.mit.edu/newsoffice/2006/engine.html
Cheers!
-Alex Dru
Free Lectures Relating to Topics from Class!
12 February 2007
Bush Doing Some Good (kinda)
http://www.senate.gov/~schumer/SchumerWebsite/pressroom/press_releases/PR02265.html
Particulate Matter in Santiago's Smog
"Where indeed? Chile's environmental review board, known as the Conama (Comision Nacional del Medio Ambiente), admits it is far behind and stretched financially on issues such as toxic waste and garbage dumps. Meanwhile, independent university experts note that forcing the purchase of catalytic cars and trucks is not the answer. Some 85% of the pollution infecting the capital is particulate matter--ground-up tire tread, leaves and dust--which hangs in the air after traffic stirs it up. The sheer number of autos and buses moving around in the city pushes pollution counts higher, not simply the volume of exhaust from their tailpipes."
The smog is so dense in the city that when I was visiting in the summer of 2005 and climbed one of the large hills that overlooked the city you could see the sea of smog lapping up against the hills and up to the edge of the Andes. Although catalytic converters are useful for reducing the emissions of NOx, as seen here in the article about Santiago, the smog problem is far reaching past one chemical process. The particulate matter that plays a central role in the smog is not identified. Chemistry can be done to specify what are the main components of the particulate matter and then address directly their sources and create solutions to the problem. The seriousness of addressing all of the factors that contribute to smog is seen here. Simply implementing the use of catalytic converters is not going to take away from the other conditions that contribute to the smog.
07 February 2007
Montreal Protocol
06 February 2007
Hydrofloroethers?!
Web address for the PDF.
http://pubs.acs.org/cgi-bin/article.cgi/jpcafh/2005/109/i02/pdf/jp047860c.pdf
05 February 2007
San Diego has a UV Monitoring Site!
02 February 2007
Human Lives affected by the Antarctic Ozone Hole?
For the full article visit: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11807429&dopt=Abstract
01 February 2007
Ozone depletion over the Arctic region
For more information on ENSO go to: http://www.research.noaa.gov/climate/t_elnino.html
on this site you can also connect to other areas of NOAA research about climates.