24 May 2007
Water Conflict and Climate Change
http://www.globalpolicy.org/security/natres/water/2007/0316thirstier.htm
http://www.netwas.org/newsletter/articles/2005/09/2
http://internationalwaterlaw.org/articles/general/Rahaman-Ganges-Asteriskos.pdf
21 May 2007
The history of DDT is extremely complex and politically connected. The chemical was developed in 1939 by Paul Muller who received the Nobel Prize for its invention as an insecticide.[1] It was used in World War II to combat typhus and malaria and was hailed as a miracle cure to diseases that previously had no such relief. In the
Much of the science that
DDT is dangerous because of its ability to bio-magnify. It is fat soluble and does not readily get released as waste. As one animal absorbs it, for example a fish through its gills, it stays there until another animal eats it that then absorbs all of it. The larger fish then absorbs all of the DDE in every little fish it eats which is then passed on up the food chain becoming more and more concentrated in the fat of each animal. For birds this is important because once a certain concentration is reached the DDE inhibits calcium absorption, and the eggshells are then too thin to support the parent birds. In the
The harm from bio-magnification is important not only where the chemical is sprayed but also globally because DDT has the ability to travel in the atmosphere. Because the chemical has a low volatility level a small percentage of it evaporates and then travels to as far as the artic where it is cold enough to condense.[8] Here the chemical can be bio-magnified in the food chain and reach humans and animals on the other side of the planet from where the chemical was sprayed. With the integration of DDE in organisms the effects are often long term and difficult to study. The most important aspect of the chemical impact is its overall harm to biodiversity. There is no way to know what organism it will harm or how, but regardless there are problems that arrive from the indiscriminate killing of organisms.
The human health risks of DDT are also complex. DDE and DDT are hormone-disrupting chemicals that can lead to immunological, developmental, and reproductive problems. There have been many studies done on humans trying to directly correlate prolonged DDT exposure to acute problems but like all carcinogens it is hard to create direct correlations to prolonged exposure because of the lack of study controls. In Tzaneen,
[1]
[2]Carson, Rachel. 1962. Silent Spring. Houghton Mifflin Company,
[3]
[4] Baird C, 450.
[5] Baird C, 445.
[6]
[7] Wiemeyer,
[8] Baird C, 414.
[9] Dalvis, Mohamed., Myers, Jonathan., Thompson, Mary Lou., Robins, Thomas., Dyerd, Silke., Riebow, John., Molekwa, Josef., Jeebhay, Mohamed., Millar, Robert., Krugerg, Phillip. 2004. “The long-term effects of DDT exposure on semen, fertility, and sexual function of malaria vector-control workers in
[10] Wolff, Mary., Toniolo, Paolo., Lee, Eric., Rivera, Marilyn., Dubin, Neil. 1993. “Blood Levels of Organochlorine Residues and Risk of Breast Cancer.” Journal of the National Cancer Institute. Vol. 85(8): 648-652, April.
14 May 2007
Chlordane awareness

Chlordane Still Present In Tecolote Canyon After Thirty Years Of Its Restricted Use
Our goal for this project was to access the locations and concentrations of Chlordane throughout the canyon and to speculate as to where the possible sources for contamination were coming from (or still are coming from). Our class was split up among various parts of the length of the canyon and soil samples were taken from numerous locations near a creek that runs through the park. At each sampling site, coordinates were taken from a GPS system that allowed us to later plug these sites into Google Earth to get a better perspective of where each sample was coming from.

From Google Earth, were were able to pin-point each concentration value for Chlordane with its respective location within the sampling area. This was a very helpful method because from the aerial view, we were able to locate possible drainage sources that might contribute to a specific concentration at a site. The samples were taken into the lab and treated with a Chlordane Kit called ELISA that was able to help us measure absorbency values from each sample. From the absorbency values compared to a standard set of absorbency values for the chemical Chlordane, the concentration for each site was able to be calculated.
Most of the calculations led to fairly low concentrations respectively, however there were a few sites that huge concentrations in comparison to the other sites. One of these sites was located at the major drainage site of the golf course, which in fact made the most sense to us. Sadly, golf courses use many chemicals to treat their grasses without paying much attention to the environment surrounding them. The other high level that was found was located near the highway where a confluence of water was located. All surrounding drainage pathways led to this one small location where the water was just stagnated. Also, when discussing this site with the local park rangers, they had mentioned that the location for that was was where a large condo-complex had been built across the street within the past twenty years and they definitely would have been using lots of pesticides to create the garden area and pest control.
Our results were higher than we would have thought, but it really hits home to know that these chemicals really do exist many years after their use. On out part, only measurements about the soil were taken into consideration, but the immense wildlife in the area definitely plays a big role into the contamination as well. It is important to think about the long term effects when using toxic chemicals and how exactly the product is getting disposed of before using them. Lastly, I would just like to point out that our tests were only run for one pesticide that was banned in the 1980's, yet still today pesticides are being used worldwide to a greater extent and it is our responsibility to make sure that we are monitoring their detrimental effects and using them minimally.
09 May 2007
Dogs Pose threat to water
08 May 2007
Constructed Wetlands
The section of the book on the processing of wastewater via an artificial marsh intrigued me to do some more research. There are two types of constructed wetlands: the subsurface - flow wetland and the surface - flow wetland. The subsurface - flow wetland transports sewage sludge through the sand to the roots of the marsh plants, while the sewage sludge in the surface - flow wetland travels above the soil. Also, there are many wetlands here in San Diego County, some of which are 'constructed' and not natural. However, the population in San Diego County is too large to have the wetlands serve as biological treatments for wastewater and sewage, which is why they were created mostly to serve as a refuge for migrating birds and to compensate lost estuarine habitat. Here is a list of the wetlands in San Diego County if anyone is interested.
06 May 2007
a Greener Apple...?


Apple has long been praised for generating products with a sleek, clean, modern design...so why doesn’t its company follow suit with respect towards its generation of "iwaste." On May 2, 2007, a bit more recent than the Clinton era ;), Steve Jobs vowed to clean up Apple' act. Apple's website now boasts it Green Apple logo and a list of improvements that will be taking place with respect to lessen its impact. These reductions include the complete phase out of lead in their computers as well as other toxic heavy metals such as cadmium, arsenic, mercury, and hexavalent chromium. The E-waste from large computer and cell phone manufacturers is gigantic. In areas of countries like China and India, computer parts and wastes are dumped in open fills where their breakdown can be slow and release these heavy metal components into the soil and ground water which can have noxious effects on the surrounding populations. Apples has also stated that by the end of 2008, they will end the use of PVC and and BFR's (Brominated Fire Retardants)--both which upon incineration can release bromine and chlorine compounds into the air. The Apple site and well as Greenpeace have picked up on this initiative by Jobs and have great full coverage. Please Visit http://www.apple.com/hotnews/agreenerapple/ or http://www.greenpeace.org/apple/ for more information.
03 May 2007
Water related Stresses of East and South Africa.


Eastern and Southern Africa (especially the Horn) have been recently been under great pressure from contamination in the welled drinking water. In these arid regions, water is obviously an extremely precious finite resource, which must be managed properly, or else great numbers of people will face imminent death. Africa's wells have recently been experiencing contamination by the microbe Vibrio cholera, a bacterium that causes Cholera. They symptoms of Cholera are dehydration, headache, stomach cramping and severe diarrhea. If a person goes untreated with various batteries of antibiotics, this bacterial infection can run havoc on your digestive system and cause death. UNICEF, a leader in humanitarian aid and Global health has identified Cholera contaminated water as an epidemic that must be fought. Stagnate conditions of water holes, combined with the equatorial heat, can turn these water sources into incubators for this microbe. UNICEF provided over 10 million dollars in recent years to work with officials in populated areas to help enact filtration methods to clean water sources, but much work is still needed. To Read more visit :http://www.unicef.org/har07/index_37421.htm
30 April 2007
Perchlorates & Hypothyroidism
25 April 2007
Bottled water vs tap water
23 April 2007
San Diego Landfills
Portland Watershed Restoration
City of Portland Adopts and Funds Watershed Management Plan
By Bob Sallinger
Warbler June 2006
On March 8, 2006 the Portland City Council adopted an innovative Watershed Management Plan that holds within it the potential to set the City on a course towards ecological sustainability. In Late March the Mayor presented a preliminary budget that underscores the City’s commitment to on-the-ground implementation of this plan. At a time when comprehensive environmental planning all to often falls victim to short-term political considerations, back-door compromises, and inconsistent follow-through, the City of Portland has demonstrated a steadfast commitment to restoring a landscape that nourishes both humans and wildlife and which preserves a legacy of green for generations to come.
In his Report to the Park Board, Portland Oregon, 1903, John Charles Olmsted wrote “Marked economy may also be effected by laying out parks, while land is cheap, so as to embrace streams that carry at times more water than can be taken care of by drain pipes. Thus, brooks or little rivers which would otherwise be put in large underground conduits at enormous public expense, may be attractive parkways.” More than a century later, the City of Portland has taken these prescient words to heart. The Portland Watershed Management Plan presents a vision in which stormwater is treated as an urban amenity rather than obstacle to progress. By simply recognizing that rain is best addressed where it falls rather than by piping it to someplace else, the City has committed itself to a new way of thinking with profound implications for the ecology, economy and livability of our urban landscape.
The Management Plan sets four watershed health goals for the City: improvement of hydrology, water quality, physical habitat and biodiversity. Based upon nearly a half a decade of work and a small mountain of scientific data, the plan ultimately does two things. First it models the entire urban landscape to determine how best to accomplish these four objectives in any specific location. The City now has a scientifically credible process for determining how to spend limited funds to best accomplish its environmental goals. Second, and perhaps more importantly, the Plan commits the city to considering and incorporating where possible watershed protection and restoration principles at the planning stage for all city projects. The Plan recognizes that the least expensive and most effective way to restore the landscape is to do things right in the first place rather than retrofitting after environmental regulations such as the Clean Water Act and the Endangered Species Act have been violated. This plan breaks down oft-criticized bureaucratic silos and makes environmental protection the purview of all city agencies.
The plan envisions an urban landscape in which problems such as flooding, water pollution, urban heat island effects, and lost of species diversity are addressed by reintegrating nature into the landscape. This includes not only traditional strategies such as the protection of parks and natural areas, but also a proliferation of street trees, ecoroofs, vegetative swales, rain gardens, vegetated curb extensions and the like.
The watershed approach makes economic sense. Commissioner Sam Adams, speaking before the Bureau of Environmental Services Citizens Budget Advisory Committee, noted that if we had implemented these types of projects thirty years ago, the city might very well not be spending $1.4 billion dollars today to address the combined sewer overflows (CSOs) that have turned our urban waterways into cesspools. If we fail to implement these types of projects now, the “big pipes’ that we are installing today will become obsolete within a few generations saddling our children and grandchildren with an even greater liability. The Portland city council signaled its recognition of this fact by not only holding the Portland Watershed Services Program Budget stable during 2006 but also creating a $500,000 Watershed Investment Fund to promote increased project implementation during the next year.
This plan is already more than simply aspirations on a page. Years of pilot projects have demonstrated that these projects make economic and ecological sense and simultaneously improve the livability of our City. To learn about projects already completed to date go to www.cleanrivers.org and view the 2005 Portland Watershed Annual Report. What the adoption and funding by city Council have done is ensure that these types of efforts will become the norm rather than the exception. Kudos to the BES Watershed Services Program, all the participating bureaus, the Portland City Council and myriad citizens who helped see this plan through to adoption.
http://www.audubonportland.org/conservation_advocacy/urbanconservation/watershedplan
17 April 2007
Taking Care of our Environment
16 April 2007
Agent Green and the US war on Drugs

Since 2000 under the Clinton administration, the US department of Agriculture was working with Colombian government officials in order to institute and implement a plan of blanket spraying 1000's of acres of rainforest in order to kill Coca (cocaine producing) plants. The main chemical being used was glyphosate, more commonly known as "roundup", which was blanketed over high yield areas in the dense Colombian rainforest. Although these areas are said to be uninhabited, it was common knowledge that not only indigenous tribes lived in these areas but also small villages existed which housed the various growers (by the way those kids are standing next to a coke plant). Because of the potential to inadvertently harm innocent people, and much to the tone of Silent Spring, the Colombian government worked to identify a natural fungus that is harmless to people but is a natural herbicide and killer of the coca plant. The fungus, Fusarium oxysporum, is being tested by the US for use and apparently is quite indiscriminate in its damage killing all types of plants. Despite its low specificity, Agent Green's use is being lobbied in both Colombia and the United States and is probably going to be used. To Read More Visit :http://www.americas.org/item_294
15 April 2007
DDT is not linked with Breast Cancer
06 April 2007
World's Dirtiest Citites
30 March 2007
Methylmercury Toxicity

Did you know the EPA has been warning pregnant women about fish consumption? Or that methyl mercury accumulates in your hair -- giving analysts a simple way to get a sample to test? A Chemical & Engineering News article summarizing mercury toxicity was published in early 2004: go to http://pubs.acs.org/email/cen/html/033007161508.html to read more. One last factoid: the Dartmouth College professor who died after exposure to a drop of methylmercury had no symptoms for 5 months after the exposure.
27 March 2007
MInimata Disease
I was disappointed in the lack of material presented about the mercury poisoning in Minamata, Japan during the 1950s. The authors of the textbook even claim, "the poisonings at Minamata must surely rank as one of the major environmental disasters of modern times", but don't quantify the disaster. According to Japan's Ministry of the Environment, "2,265 individuals in Minamata and the surrounding area have been inflicted with mercury poisoning from the discharge of industrial waste water and 10,000 individuals are being compensated for their exposure and loses". Not to mention, the Japanese government and the Chisso corporation responsible for the mercury industrial waste have paid billions of yen to dredge the sediment containing the mercury and also to develop education programs about mercury poisoning. The picture really shows the horrible crippling that the body undergoes when exposed to toxic levels of methylmercury.
21 March 2007
Oh no, no mo Rice!

I was struck in Chapter 8 when I read that there was an increased incidence of pulmonary adenocarcinoma (lung cancer) in Chinese women due to prolonged exposure to cooking-oil fumes. There have been numerous documented stories and research which has affirmed the presence and contribution of Polynuclear Aromatic Hydrocarbons (PAH's) to this phenomenon. This really hit home for me not only because of my love on chinese food and general concern for those who prepare it, but also my love of cooking. Have I been over exposing myself to high levels of PAH's in cooking oils and products all in search of that one delightful recipe? According to published work, the answer is yes. Although the names of the brands of synthetic oil were not revealed in these studies, the respirable cooking oil particulate is quite dangerous and prevalent. Ways to prevent high exposure are to take such cautionary measures such as working in well ventalated areas and to always cook with the blowfan on. Also whenever possbi;e try to avoid high oil temperatures because this serves to increase the amount of oil PM. For more, read this; http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8161241&dopt=Abstract.
20 March 2007
Plant Buddies
18 March 2007
San Diego's Greenhouse Gas (GHG) Footprint Group #1


- Reduce the driving week by encouraging people to drive only six days per week instead of seven.
- Encourage the automotive manufacturers to increase or have government mandated miles per gallon ratings to improve the miles per gallon a vehicle gets.
- Replace natural gas with renewable energy such as solar or wind power.
- Encourage people to join a carpool.
- Give incentives for people to own hybrid vehicles by giving tax cuts to encourage people to replace their old cars with hybrid models.
- Give incentives and more protection on the road to encourage people to buy and drive motorcycles instead of cars.
According to an article from the Union Tribune, the goal of reducing GHG emissions to 7% below the 1990 levels maybe impossible. Two solutions mentioned would require more than half of the vehicles be removed from the road or eliminating all residential and industrial energy use in the city. Of course these solutions are unrealistic but unless something drastic is done to reduce the GHG emissions, the emissions will continue to increase with the growing population of San Diego. From studies, the reasons for the increase in emissions from 1990 to 2004 were due to an increase in computer rooms that must remain cool, energy use from residential homes, and vehicles on the roads. Many of the people are optimistic because city residents might not be willing to make significant changes to their daily lives to lower the GHG emission. In order for emission reduction to happen education of the public has to happen to make people aware of the severity of global warming and the city needs to make incentives for people to want to change their daily lives to better the environment.
Artificial Trees: A Good Solution?
 Jumping back into our Monday discussion about greenhouse gases, I mentioned a website I had found that talked about artificial trees. I actually went back and found the website that was talking about these "trees" and the article did sound convincing to me. Although the name trees might be deceiving, these devices look more like windmills than actual trees. Apparently, these "trees" individually can remove 90,000 tons of CO2 per year, which is equivalent to emissions of CO2 by15,000 cars. That sounds pretty amazing to me, considering the largest contributor to CO2 emissions are cars themselves. I do want to mention that these "trees" aren't perfect, they do take energy to collect the CO2, but it would create a renewable source of CO2 for synthetic gasoline.
Jumping back into our Monday discussion about greenhouse gases, I mentioned a website I had found that talked about artificial trees. I actually went back and found the website that was talking about these "trees" and the article did sound convincing to me. Although the name trees might be deceiving, these devices look more like windmills than actual trees. Apparently, these "trees" individually can remove 90,000 tons of CO2 per year, which is equivalent to emissions of CO2 by15,000 cars. That sounds pretty amazing to me, considering the largest contributor to CO2 emissions are cars themselves. I do want to mention that these "trees" aren't perfect, they do take energy to collect the CO2, but it would create a renewable source of CO2 for synthetic gasoline.
17 March 2007
San Diego's Greenhouse Gas Footprint Group #2
Have you ever considered how your city and even you could reduce carbon dioxide emissions? In
Widespread electrical conservation included turning off computers when not in use and unplugging appliances in the home while not in use. Many suggestions were included in the transportation wedge including biking, walking, raising gas prices, and adding incentives for carpools. Also purchasing cleaner 4 stroke marine engines would limit carbon dioxide emissions from transportation vehicles. Environmentally economical zoning and building purposes high density housing and living close to work as options for lowing carbon dioxide emissions. Two other suggestions focused on increasing laws and knowledge about carbon dioxide emissions. For example, people in
In an article entitled, “Alter climate?
13 March 2007
Trace chemicals and reproductive health
Red Cross Symbol on Pesticide Products
11 March 2007
Be Good to the Planet or Be Good to Yourself?
It couldn't have been better timing for a greenhouse gas article to close up chapter 6 while also incorporating chapter 7 on pesticides. Since organic produce isn't always local, most likely it had to be flown in from across the country or even equator, thus contributing to the addition of greenhouse gases to our atmosphere. Pretty much the act balances itself out. The articles main focus was to buy local. Even if the produce isn't organic, it hasn't been boxed up an shipped all around the world, just so someone can have an organic pineapple.
Time reporter Gussow's famous statistic is that "Shipping a strawberry from California to New York requires 435 calories of fossil fuel but provides the eater with only 5 calories of nutrition."
It makes you wonder if that strawberry is really worth it.
07 March 2007
Biomagnification of DDT
We attempted to measure the global warming potential (GWP, a relative measure of how effective a particular gas is at trapping infrared light, with carbon dioxide having a GWP of 1) of several laboratory solvents and gases, including CH4, CHCl3, CCl4, CH2CL2, CO2, and a halon mixture. Analyzing trends across these different gases would hopefully allow us estimate the impact of these gases on global warming at the rates they are currently being released as well as to make predictions as to the GWP’s of related gases.
 (Fig1: Static Dilution Setup, Seen from the side)
(Fig1: Static Dilution Setup, Seen from the side)
(Fig2: Flowing Dilution Close-up)
In order to accomplish this, we took dilutions of these gases and measured them using Fourier Transformed Infrared Spectroscopy (FTIR). Whenever a gas must first have been vaporized from a liquid, a static dilution system was used to allow us to obtain quantitative dilution. We used a much more simple flowing dilution system whenever a gaseous source was available for us to make our dilutions from. The absorbancies were then collected and measured using a salt crystal FTIR collection cell. At this point the treatment of the data became highly mathematical. The wavelengths and intensities at which each molecule would absorb infrared radiation were entered into an equation that would give us out the GWP of that particular gas.
When we compared the GWP’s that we experimentally derived to “standard” values that have been tabulated already, we found that our results were vastly (about 10x-20x) lower that the published values. A quick glance at fig. 3 shows this. If we had gotten experimentally reasonable data, the bar graphs for each particular gas would match or at least be reasonably close. One likely culprit was the dubious security of air locks on the FTIR cells. If these locks were in fact leaky, then we were measuring gas concentrations that were significantly lower than what we were expecting to measure. Placing the larger concentration into the equations instead of the correct but unknown smaller concentration caused by the leaky cell would have given us this deviation. Other possible causes were incorrect use/calibration of the FTIR specs or human mistakes that took place in the dilutions.

Even though most of our data points were less than accurate, we were still able to make connections between some of the chemicals we use on a daily basis in the lab and the global warming effects we see increasingly each year. At the very least we were able to see that in fact, yes, greenhouse gases do absorb thermal radiation as well as the fact fact that some gases are more efficient in this action than others. Since these gases only represent a very small portion (excluding methane) of the GHG’s, we in fact only studied a small portion of the global warming effect. More research is needed into the entire field is needed not so much to discover more of the chemistry involved but more so into effective education techniques, for each day an individual does not fully understand his or her impact on the environment, the closer we get to changing our planet in a way we can’t fix.
Greenhouse warming potentials
the results are as follows
Gases Experimental Actual
Methane, Group1 1.349 23
Methane, Group2 2.798 23
Chloroform 0.0459 5
Freon TF 1.589 1
CH2Cl2 0.590232 9
CCl4 3.770566 1400
Carbon Dioxide 1
From the data collected it is obvious that the gases yielded very low values for the GWP of the gases. Despite that, the values did reflect the general trend of actual GWP values relative to each other with the highest being CCl4 having a value of 3.8 followed by methane with an average of 2.1 and chloroform being the least at 0.05. The halon mixture did not follow the GWP trend but had the lowest percent error.
In conclusion, we may not have found all the answers to global warming but from this lab we were able to predict what gases are contributing more to the greenhouse effect.
Hydrogen Fuel Concept Cars

Obviously on of the biggest hindrances in Hydrogen being incorporated into passenger vehicles is in its storage. Whether a metal hydride is used or pure liquid hydrogen, every phase presents its own problems. This is why large car manufactures such as GM, and individual brands like BMW have been working on safe, compact storage designs for the hydrogen energy. The concept cars they have drawn up either contain a tank for the hydrogen fuel within the car, or it is fully integrated into the chassis of the car. It is important to realize that when solving problems such as future fuels that although the chemistry of the process is extremely important, that the practical engineering and integration of the ideas cane be just as difficult to tackle. For more, read this issue of Popular Mechanics at http://www.popularmechanics.com/automotive/new_cars/1266796.html.
06 March 2007
Solar Energy in La Mesa
28 February 2007
Global Warming??
26 February 2007
Thermohaline Circulation Shutdown!?!?
21 February 2007
The Global Warming Debate.
Personally, my standpoint on the whole global warming debate is that, the general public already knows that global warming, is essentially just that. I think that the focus of the media and the government should be focused on educating the general public on exactly how, as an individual, one can help the situation out. For example, in the movie Al Gore kept giving horrifying statistics and facts, but didn't really offer a way to connect a reaction that I think would be important to our main goal--to reduce greenhouse emissions. Whether or not the facts are exactly true or whether the predictions made by scientists are fully supported, there is the chemistry behind all of our daily processes that does go to show that their worries stem from something.
While I was looking online to find some of the opposing views to the Global Warming hype, I stumbled across this article written by Capitalism Magazine about how the public is forced into accepting the views that the scientists and media has due to the fact that no one will allow a public disclaimer about how the global warming hype might not be as important as we originally thought. It is very interesting to hear other sides to the story, especially from someone who is not a scientist and only a journalist, but those people are out there.
19 February 2007
Carbon Dioxide Bubbles in Ice Cores

Chapter 4 mentions constructing past carbon dioxide profiles from the air trapped in ice cores, which I thought needed more explanation. Ice forms as new snow accumulates on top of old snow increasing the pressure. The increased pressure forms the crystalline lattice, which consists of open air spaces where carbon dioxide can be trapped during the time period of formation. With the aid of dating techniques, scientists can date each ice deposition and determine the amount of carbon dioxide present at that time period. The graph above shows how the amount of carbon dioxide and aerosols in the atmosphere was correlated to temperature (higher carbon dioxide concentrations equals higher temperatures) for the Vostok Ice Core in Greenland. For more information on ice cores and their use as paleoclimate proxies visit this page.
What do you do while driving? It may be more harmful than you think. Transportation devices as a whole still contribute almost half of the nitrogen oxides (NOx) introduced into the atmosphere today. Nitrogen oxides contribute to acid rain, the greenhouse effect, deterioration of the ozone layer and smog. The catalytic converter present in most modern cars changes nitrogen oxides back into nitrogen and oxygen using unburned gasoline. One downfall to these catalytic converters is that they only work on warm/hot engines. This means that, once a car is started, until the time the engine is “warm”, the tailpipe is essentially, emitting high levels of pollutants to the environment.
In this lab, vehicle exhaust is tested for both nitrogen oxides and particulates when the car is cool and when the car is warmed up. Since NO2 absorbs visible light, standard calibration curves for NO2 and nitrogen absorbing solutions were made. Also, using a SMPS system, that measures particulates in the air, particulate output and NOx output were assessed for seven different vehicles.
The results are as follows. From our results of hot vs. cold starts, we were expecting to find higher concentrations of NO2 on “cold starts” for each car, and lower concentrations for “hot engines”. It is interesting to note that the cars do not all exhibit the same behavior over time (the cool exhaust contains less NOx, or vice versa). While on day two of collecting data from cars, 3 of the 4 cars tested did follow our predictions (the 4th car however was the extremely old corolla, most likely not having a catalytic converter), almost the entire opposite trend happened on the first day of car sampling. Therefore according to our data, no exact conclusion could be drawn regarding the catalytic converter assumption that lower emissions of NO2 would result from a warm engine. In order of highest to lowest cold start NOx emissions the cars are as follows: Jetta, Corolla, Civic, Villager, Saab, Alero, and Echo. As for the hot, the cars rank Corolla, Saab, Villager, Alero, Civic, Echo, and Jetta. The Villager, Jetta and Civic produce much lower concentrations once they have warmed up, while the Corolla almost appears to have a malfunctioning catalytic converter.
The data from the SMPS was also analyzed and graphed, showing total particle numbers and particle volumes.
All the cars except the Jetta and Civic have greatly reduced particulate emissions once they are warm.
In conclusion, it seems that the production of nitrogen oxides in vehicle emissions is very unpredictable. However, once cars are warm they tend to greatly reduce their particulate emissions.
indoor air pollution
Vehicle Emissions Experiment 2-12-07/2-14-07
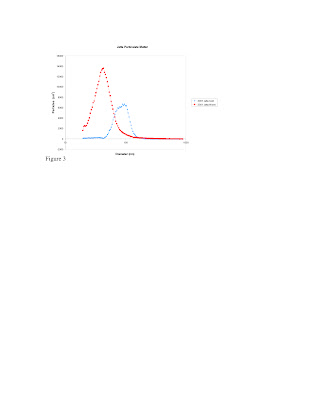

When a cars engine runs it reacts N2 and O2 during combustion under high heat creating NO as a by product. NO is a harmful chemical to be released into the air because it can undergo further chemical reactions leading to NO2. Together, NO and NO2 are referred to as NOx. These chemicals are important because they are involved in numerous photochemical reactions that lead to the creation of low altitude ozone O3, which is a primary component of smog. NOx can also react to create acid rain. According to the EPA (2003), vehicles contributed to 55 % of the total man made source of NOX emissions (http://www.epa.gov/air/urbanair/nox/index.html). We all drive cars, so each of us has a level of responsibility in creation of smog and acid rain; it is important to us to understand just how much Nox is being released by our cars.
Particulate matter is also created by the engines mechanical processes and released in emissions. Particulates also contribute to smog and therefore contribute to health problems correlated with this phenomenon. Some studies have even correlated increased levels of smog to death rate (Baird and Cann, 2005). The sizes of particles are important because the small, man made particles are inhaled directly into lungs; our bodies are not adapted to processing or filtering them or otherwise protecting us. Larger particles can be trapped by our bodies, and are less harmful to the environment because they do not stay in the air as long before settling out. The size and the amount of particulate matter that is released by our vehicles was also a focus of the study.
In order to investigate these two common pollutants from car emissions we conducted an experiment to identify the concentration of NOx and the size and concentration of particulate matter in seven different car models. The cars that were tested ranged in age from 1979 to 2001 and included small models such as a Corolla and Jetta. The NOx was tested by collecting air samples directly from the tail pipe of the running cars. Air samples were mixed with an NO absorbing solution that then reacted to give us a measurable amount of color. A UV vis-spectrophotometer was used to measure this difference in color. By creating a set of standard solutions with known concentrations that were measured by the UV vis- spectrophotometer a calibration curve was calculated. The concentration of the NOx in the car emissions could then be visually compared to the standard solutions and a concentration was calculated in units of parts per million (ppm). This tells us how many particles of NOx there are in relation to all the particles in the air. The particulate matter was measured separately using a Scanning Mobility Particle Sizing (SMPS) device. This device calculates the concentration and size of particulate matter using several pieces of machinery and a computer.
Figure 1 illustrates NOx concentration data from four of the cars that were tested. We observed that total NOx generally starts out at a relatively high range, and decreases for most cars after warm-up. This trend likely results from the fact that all cars that we tested use catalytic converters designed to reduce emissions, which do not begin to work until the cars are warm. Newer cars were built under emission regulations that have become increasingly more stringent. This is supported by the data that showed overall NOx was the highest in older vehicles and the lowest in newer vehicles.
The data from Scanning Mobility Particle Sizing (SMPS) system of the 2001 Jetta and the 1979 Corolla (Figure 2) show the amount of particles at the range of sizes from 0-1000 nm in diameter. The important thing to notice is that for the Corolla the majority of particles are released before the car has warmed up and before the catalytic converter starts to work. For the Jetta the opposite is true, the majority of the particles are released after the car has warmed up. The sizes of the particles are slightly smaller also in the Jetta than the 100 nm particles released during the cold start of the Corolla. The regulation of large particulate matter in the newer Jetta might be allowing for the smaller particles to be released and go less noticed.
All of the data helps us to make observations about the composition of car emissions and connect it directly to creation of air pollution. As the world continues to modernize man made pollutants are becoming more noticeable and problematic. Specifically, increased vehicle emissions of NOX and particulates are polluting most moderately sized cities. Understanding how cars release these harmful pollutants increases our awareness of the issue and hopefully will help us to create future solutions
14 February 2007
Are We Really Conscious of the Air We Breath?
As I was reading my weekly Time Magazine, this week had an article on "Greenhoouse Airlines" and how 1.6% of greenhouse gases is due inpart to airplanes. It was interesting because it also mentioned that although this was a small percentage, the carbon released was at higher altitudes than ground level and thus had a greater warming effect. Apparently, long flights are equivelent to months of driving SUV's. That's somehting that I am sure not many people have thought about. The article also mentioned how the Prince of Wales made a public effort to cut back on his flights last year. It just makes me wonder how many more people might cut back on their flights if they were made conscious of the decisions they make.
Time Magazine, "Greenhouse Airlines" by Brian Walsh; February 12, 2007
www.time.com
13 February 2007
Pint-sized car engine
I mean if that doesn’t sound interesting then maybe some of the stats will blow your mind. Like that this new engine will go 30% farther on a gallon of fuel than a typical gas engine. And, if all cars had this engine today, we would be using 30 billion gallons of gasoline..yea GASOLINE, NOT OIL. Also, the emissions would be much cleaner due to the ethanol supplementation. That’s huge!
Read up on this at the following website: http://web.mit.edu/newsoffice/2006/engine.html
Cheers!
-Alex Dru